Fourth dose COVID vaccine
People ages 12 years and older who are moderately or severely immunocompromised should receive a total of 4 doses of mRNA COVID-19 vaccine to stay up to date. Lela Moore is a freelance journalist whose.
Fourth Dose Of Covid 19 Vaccine Reduced Death Among Hospitalized Patients During Omicron Wave In Israel
Updated guidance that the following people should receive a second COVID-19 booster dose.
. Funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Cla. Ad For a full list of ingredients please see the CDC fact sheet for each COVID vaccine. New guidance for use of a Pfizer-BioNTech COVID-19 Vaccine booster dose in children ages 511 years.
Access expands for fourth covid vaccine doseA FURTHER 15 million Australians will be eligible. A fourth dose of Moderna or PfizerBioNTechs mRNA Covid-19 vaccine -- which is already authorized for people 50 and older in the United States -- seems safe and provides a substantial boost to. Four doses was 1801 cases per 100000 persons 95 CI.
A fourth dose of a COVID-19 mRNA vaccine can boost antibodies and other immune responses to levels higher than those seen after the third dose according to UK trial data. The Food and Drug. None of the authorized or approved vaccines contain the live virus that causes COVID.
Heres what you need to know about the winter booster program. While protection from a third COVID-19 vaccine dose seems to wane after four months experts arent so sure that a fourth dose will be necessary for all. In days 7 to 30 after a fourth vaccine dose the difference in the absolute risk three doses vs.
A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19-related outcomes among persons who had received a third dose at least 4 months earlier. This includes eligible children aged five to 11 years old. Americans 50 and older can get a second COVID-19 booster if its been at least four months since their last vaccination.
This includes eligible children aged five to 11 years old. March 29 2022 -- The FDA said Tuesday that it approved fourth doses of COVID-19 vaccines for many Americans to protect the most vulnerable people against severe COVID-19 illness hospitalization. The 4 doses include a primary series of 3 doses of an Pfizer-BioNTech or Moderna COVID-19 vaccine plus 1 booster of an Pfizer-BioNTech or Moderna COVID-19 vaccine 4th dose.
British Columbia is offering a fourth dose of COVID-19 vaccines to seniors starting with residents of long-term care and assisted-living. The CDC recommends that moderately or severely immunocompromised individuals who received a two-dose mRNA COVID-19 vaccine get an additional primary vaccine. About 3 percent of US.
Some groups of people will be able to receive their fourth and in some cases their fifth dose of a COVID-19 vaccination. The top US. The nations expert vaccine advisory group has recommended another 15 million Australians get a fourth COVID-19 vaccine.
To help fend off another wave of Covid-19 people will need a fourth dose of vaccine Pfizer CEO Albert Bourla told CBS on Sunday. Protection against infection waned however after 5 weeks but not. Eligible immunocompromised individuals aged 12 and over can receive a fourth dose first booster after their third dose.
Some individuals who are immunocompromised can get a third dose of the COVID-19 vaccine eight weeks after their second dose as part of an extended primary series. A fourth dose of Pfizers COVID-19 vaccine provides additional protection against coronavirus infection and severe COVID-19 outcomes compared with three vaccine doses but. Countries are beginning to offer a fourth dose of the Covid-19 vaccine to vulnerable groups but medical professionals are undecided on whether it would benefit the wider population.
Adults or about 7 million people are immunocompromised. Immunocompromised People Should Get a Fourth Shot. Said certain people who received the Moderna or Pfizer.
People ages 50 years and older. Many variants are coming and Omicron was the first one that. The expanded eligibility is for people with immunocompromised conditions.
Israeli health officials have gone one step further. 22 as cases and hospitalizations crept upward the country authorized a fourth dose of the Pfizer-BioNTech mRNA vaccine for all health. The 05 mL 100 micrograms dosage is used for all three doses of the primary series for immunocompromised patients to mount a sufficient initial immune response but the lower booster dose of 025 mL 50 micrograms is recommended for boosters based.
Compared with a third vaccine dose a fourth dose of the PfizerBioNTech COVID-19 vaccine lowered the risk of infection symptomatic infection hospitalization severe illness and death 52 to 76depending on the measureamid the Omicron surge among older adults finds a new Israeli study. A fourth dose of the BNT162b2 vaccine was effective in reducing the short-term risk of Covid-19-related outcomes among persons who had received a third dose at least 4 months earlier. People ages 12 years and older who are moderately or severely immunocompromised.
Health regulators on Tuesday cleared fourth Covid vaccine doses for older adults amid uncertainty over whether an even more contagious version of omicron will cause another wave of. A fourth dose of a COVID-19 vaccine restores antibodies to levels observed after the third dose but provides only a modest boost in protection against infection according to a small trial carried. Researchers recruited 166 adults who had received a booster dose of the PfizerBioNTech mRNA vaccine after two doses of either AstraZenecas viral vector vaccine or initial.
Eligible immunocompromised individuals aged 12 and over can receive a fourth dose first booster after. Moderately and severely immunocompromised people aged 18 years who completed an. After the fourth dose both messenger RNA mRNA vaccines induced IgG antibodies against the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 receptor-binding domain and increased.
For the Moderna vaccine the fourth booster shot will be half the dose from the primary series.
Israel Mulls Offering 4th Covid Vaccine Dose To All Adults Reuters

Covid Vaccines It S Too Early To Consider Giving People A Fourth Dose Say Eu Officials Euronews
/cloudfront-us-east-1.images.arcpublishing.com/gray/COIGUL3PCND75MUK3PX7C4UQGE.jpg)
Uva Doctor Explains Who Qualifies For A Fourth Dose Of The Covid Vaccine

Coronavirus Digest Fourth Jab Not Enough To Fight Omicron Study Shows News Dw 17 01 2022
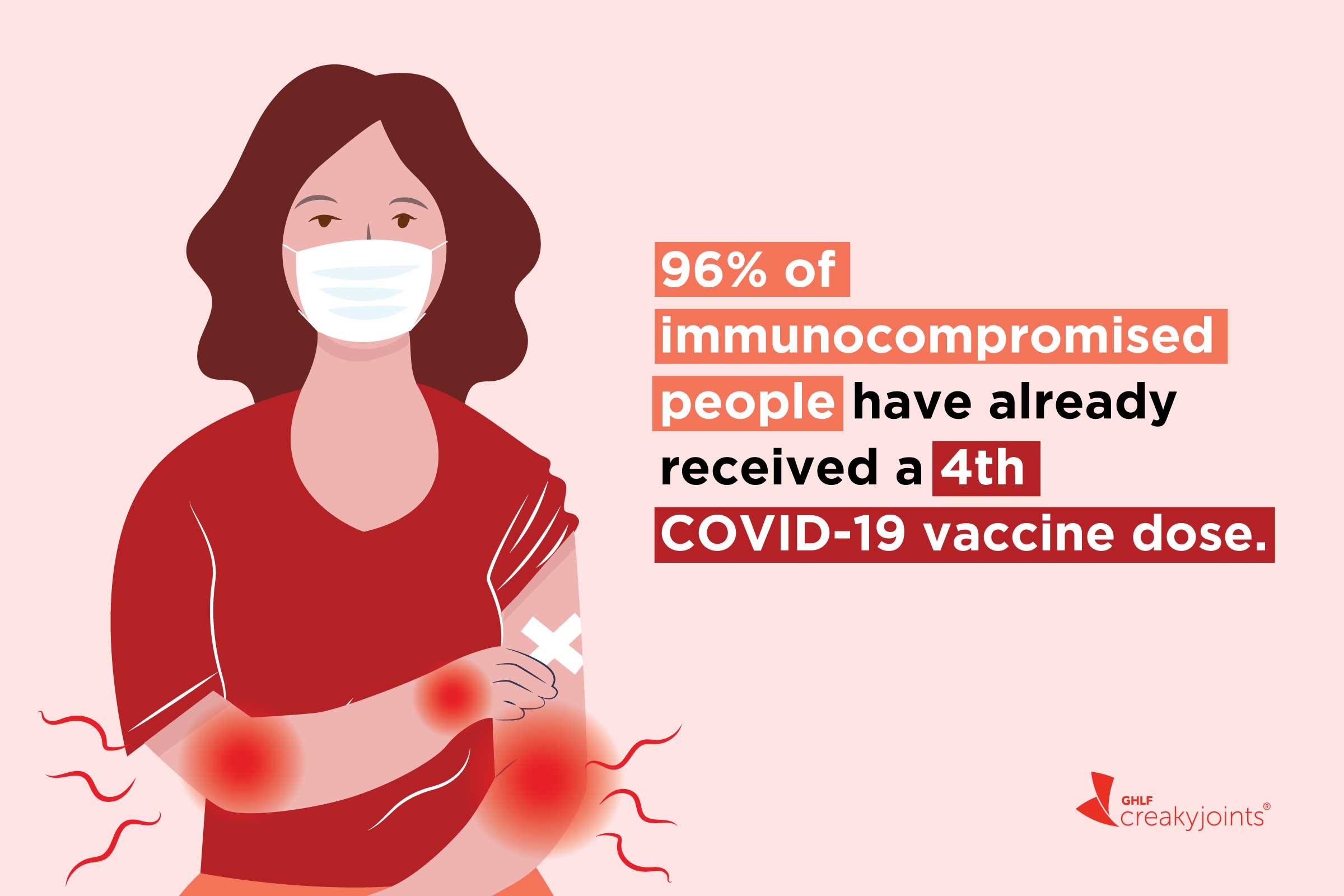
Most Immunocompromised People Have Gotten The 4th Covid 19 Vaccine Dose

Cdc Some Immunocompromised People Can Get A Fourth Dose Medpage Today

Do I Need A Fourth Dose Of The Covid 19 Vaccine Chccc

Chile A Vaccine Front Runner Launches Fourth Covid Dose Reuters

Cdc Recommends Fourth Pfizer And Moderna Covid Vaccine Doses For People Age 50 And Older

The Elderly May Need Fourth Dose Of Covid Vaccine Says Expert The Independent

Pfizer Asks Us To Allow 4th Covid Vaccine Dose For Seniors

Will States Offer 4th Covid Vaccine Doses West Virginia First To Ask Cdc For Permission

Fourth Dose Of Covid Vaccine Offers Only Slight Boost Against Omicron Infection

Should You Get A 2nd Covid Booster The New York Times

With Covid Cases Surging Across Australia Will A Fourth Vaccine Dose Be Required Coronavirus The Guardian
Israeli Trial World S First Finds 4th Dose Not Good Enough Against Omicron The Times Of Israel
/cloudfront-us-east-1.images.arcpublishing.com/gray/NY6G5YPNTRBL7FKBLYD7OXLTGA.jpg)
Fact Finders Who Is Eligible For The Fourth Dose Of The Vaccine